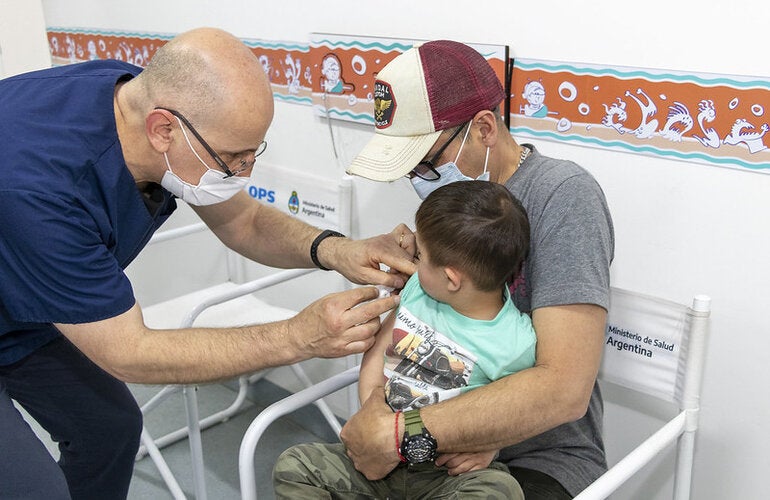
Medicines and vaccines have transformed the prevention and treatment of diseases over time. In addition to their benefits, they can have side effects, some of which may be undesirable and/or unexpected. Pharmacovigilance is the science and the activities related to the detection, assessment, understanding, and prevention of adverse effects or any other medicine or vaccine related problem.
The pharmacovigilance seeks to ensure that the benefit-risk ratio remains favorable throughout the life cycle of amedicine , i.e., from the time it is authorized until it is withdrawn from the market, or its production is discontinued. Pharmacovigilance comprises various activities involving the management and analysis of public health risk to ensure the rational use of medicines. Risk identification, quantification, and assessment associated with the use of medicines can avoid or minimize harm to patients and can prompt the adoption of necessary measures, including any necessary regulatory actions.