Guideline in development
Proposed title
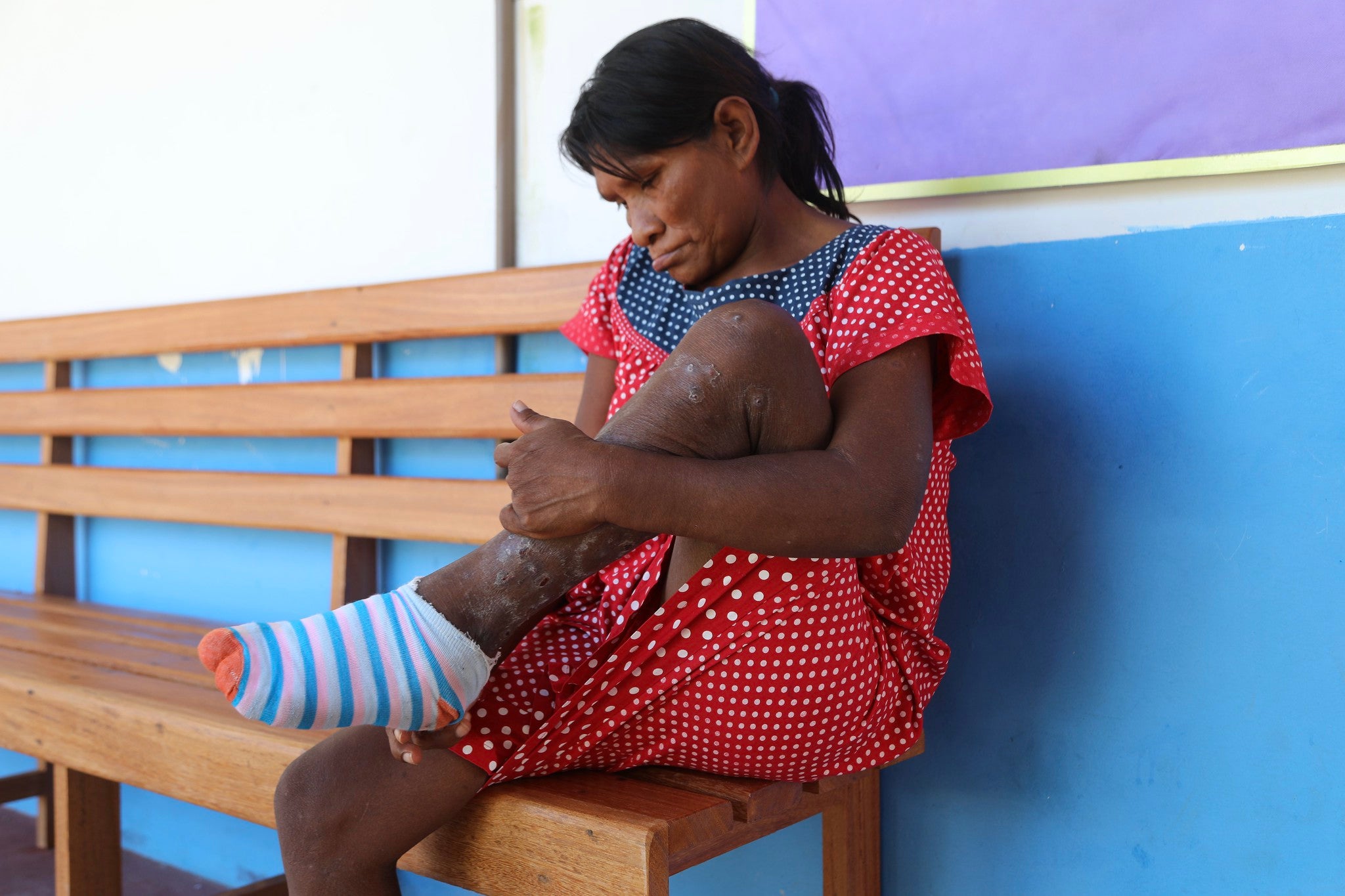
Tungiasis treatment guideline
Purpose and specific objectives of the guideline
No tungiasis treatment guidelines have been published to date. However, in the last few years, new treatments have been tested, with some promising new treatments emerging. Therefore, there is a need to develop guidelines.
This guideline will be used to make recommendations for tungiasis treatment. The objective is to make available to healthcare professionals in countries where populations are affected by tungiasis recommendations on the treatment and prevention of tungiasis based on new evidence of therapeutic interventions. With the aim of reducing the number of severe forms of tungiasis and decreasing the burden of this neglected disease. With this guideline, PAHO/WHO will be able to fulfill its normative role and provide clear and evidence-based guidance to countries and their partners.
Estimated timeline
Expected publication April 2024.
Contact
Dr. Martha Saboyá at saboyama@paho.org
Dr. Santiago Nicholls at nicholls@paho.org
Systematic review team
Ignacio Neumann, Director, GRADE Cono Sur; Universidad San Sebastian; Santiago de Chile, Chile.
Eduardo Quiñelen, Universidad San Sebastian; Santiago de Chile, Chile.
Paula Nahuelhual, Universidad del Desarrollo; Santiago de Chile, Chile.
Tungiasis treatment guideline
Below are the names and short biographies of proposed members of the Guideline Development Group.
DISCLAIMER
In order to enhance its management of conflicts of interest as well as strengthen public trust and transparency in connection with PAHO/WHO meetings and activities involving the provision of technical/normative advice, the names and brief biographies of individuals (“Published Information”) being considered for participation in a PAHO-convened Guideline Development Group are disclosed for public notice and comment.
The Published Information is provided by the experts themselves and is the sole responsibility of the individuals concerned. PAHO is not responsible for the accuracy, veracity, and completeness of the Published Information provided. Furthermore, in no event will PAHO be responsible or liable for damages in relation to the use of, and reliance upon, the Published Information.
The comments received by PAHO through the public notice and comment process are treated confidentially and their receipt will be acknowledged through a generic email notification to the sender. Comments brought to the attention of PAHO through this process are an integral component of WHO’s conflict of interest assessment process and are carefully reviewed. PAHO reserves the right to discuss information received through this process with the relevant expert and disclose to this expert the name and affiliation of the provider of such information. Upon review and assessment of the information received through this process, PAHO, in its sole discretion, may take appropriate management action in accordance with its policies.
Guideline Development Groups provide technical and/or normative advice and recommendations to PAHO. Participation in a Guideline Development Group convened by PAHO does not necessarily mean that the views expressed by the expert concerned are shared by PAHO and/or represent the decisions or stated policy of PAHO.
The list of participating experts, a summary of relevant interests disclosed by such experts, and any appropriate mitigation measures taken by PAHO relating to the management of conflicts of interests, will be reported publicly in accordance with PAHO/WHO policies.
Please send comments to Dr. Martha Saboya saboyama@paho.org