Influenza vaccine
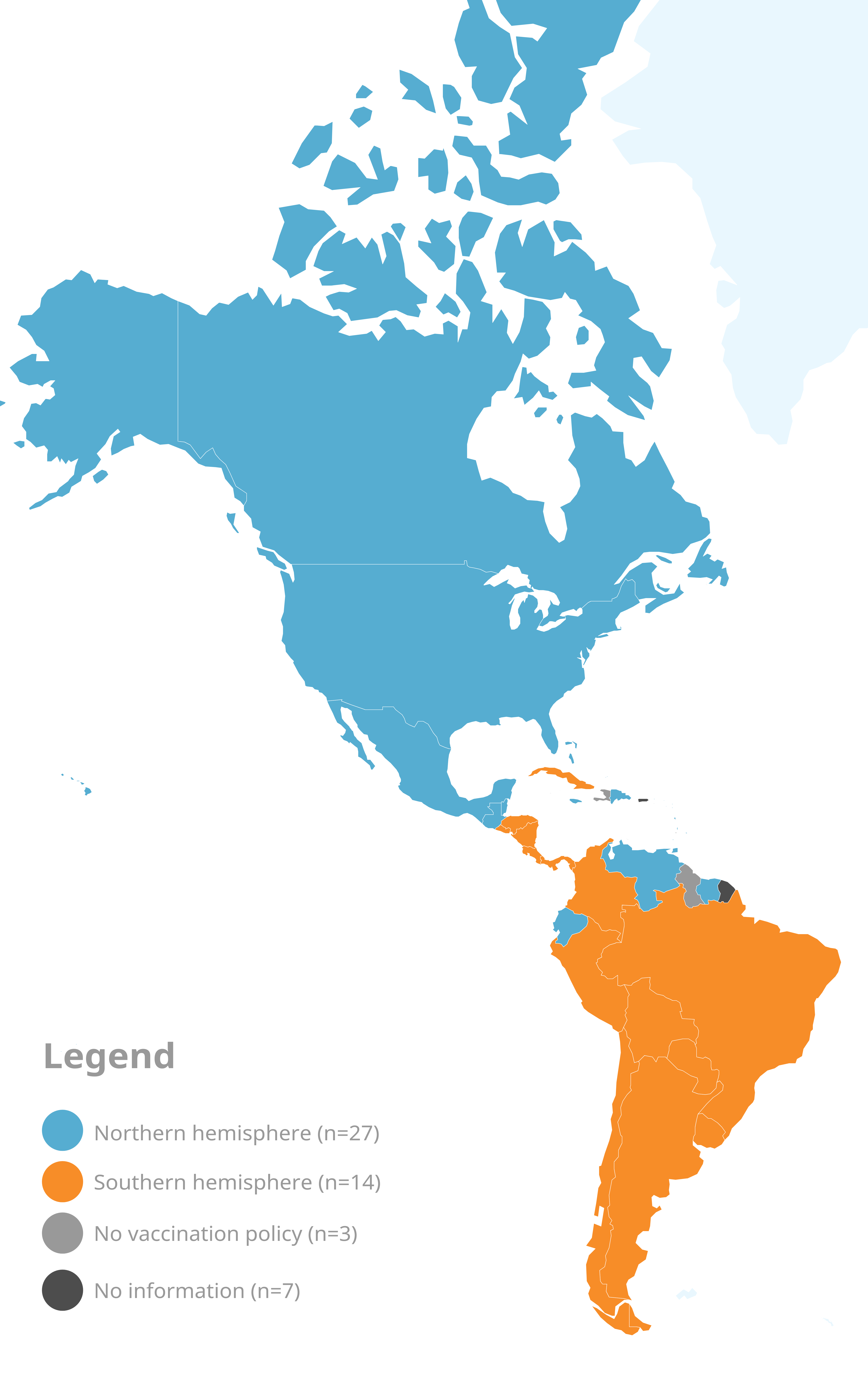
The main purpose of seasonal influenza vaccination is to avoid severe disease from infection with the influenza virus. Currently, 41 countries and territories in the Americas offer influenza vaccination to nationally-defined high-risk groups.
WHO position papers on Influenza vaccine
Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean - influenza, (REVELAC-i)
Mandates
PAHO's Technical Advisory Group on Vaccine-Preventable Diseases (TAG) recommended that all countries should establish a seasonal influenza vaccination policy that seeks to vaccinate:
- children 6-23 months of age,
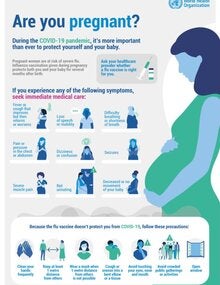
- pregnant women,
- individuals with chronic illness,
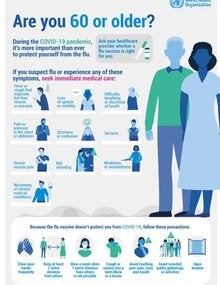
- older adults,
- and health workers.
PAHO’s technical cooperation aims to:
- Support generation of disease and economic burden estimates and leveraging REVELAC-i vaccine effectiveness and impact studies to inform decisions;
- In collaboration with the PAHO Revolving Fund facilitate rapid access and distribution of seasonal influenza and pandemic vaccines;
- Build vaccine awareness and confidence to increase influenza vaccine uptake in high-risk groups;
- Ensure continuous learning through documentation and sharing of lessons learned and best practices.
SEASONAL INFLUENZA
PANDEMIC INFLUENZA
- Technical guidelines for vaccination against the pandemic influenza virus
- Response to Pandemic (H1N1) 2009 in the Americas: Lessons and Challenges
- GAP: Guidance on development and implementation of a national deployment and vaccination plan for pandemic influenza vaccines
- Template: Plan of Action and Budget Pandemic Vaccination
- Course "Pandemic Influenza Vaccines: National Deployment and Vaccination Plans"
- Ten threats to global health 2019
COVID-19 INFLUENZA VACCINE
COMMUNICATION MATERIALS
Other Multimedia Resources from WHO