Cold Chain
Welcome to the Cold Chain Resource Center
PAHO’s Immunization Unit welcomes all health workers, EPI managers, the managers with responsibility for cold chain and supply chain operations, and the general audience to our website.
Today’s immunization cold chain and supply chain operations have been one of the key elements in expanding the provision of daily immunization services and allowing more people to be protected from vaccine-preventable diseases. When the PAHO Immunization Unit was established in 1977, both PAHO and national program staff understood that the immunization cold chain was the dorsal column of the program.
To assure that vaccines provide the expected benefits when a patient receives an immunization, epidemiologist and health staff understand that each dose of vaccine administered has to be potent for protecting the recipient against the targeted disease(s). To achieve this objective the Immunization Unit, in PAHO, focused on five pillars:
- advising countries to use good quality refrigeration equipment;
- training of health staff in managing vaccines and their equipment;
- carrying out evaluations on cold chain and supply chain operations;
- carrying out research and development in the area of cold chain technology – both soft and hard;
- improving management capacity and skills to support all operations related to the cold chain and supply chain, to introduce new vaccines.
Key Achievements
Operations of the Cold Chain
WHO: Immunization Supply Chain and Logistics
WHO Performance, Quality and Safety (PQS)
Resources
Effective Vaccine Management (EVM)
Related links
PAHO: Immunization
FAQs
Q 1 – What is the cold chain?
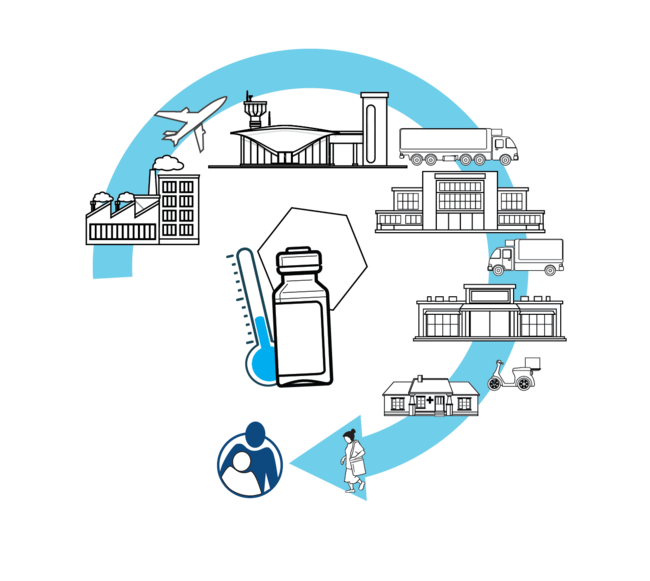
The cold chain is a set of rules and procedures that ensure the proper storage and distribution of vaccines to health services from the national to the local level. The cold chain is interconnected with refrigeration equipment that allows vaccines to be stored at recommended temperatures to maintain their potency.
Q 2 – What is the supply chain?
The supply chain is the distribution of vaccines and other immunization program inputs that follow a schedule of shipments established to ensure that each health facility receives its vaccines and inputs at the right time, in the right amount, in the right conditions and temperatures.
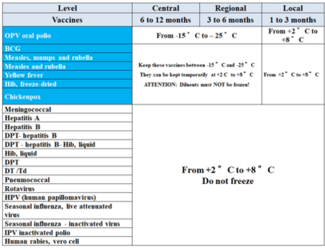
Q 3 – What are vaccine storage temperatures?
Depending on the type of vaccine there are two temperature ranges for storage: Vaccines that are sensitive to freezing should be stored at temperatures between 2°C and 8°C. Vaccines produced with viral and/or lyophilized strains can be stored at temperatures between -15°C and -25°C. The following table summarizes vaccines, storage period, and temperatures according to the operating level of the cold chain:
Q 4 - When using completed frozen water-filled ice packs to pack a container, how does one prevent the freezing of vaccines which are freeze sensitive?
Before introducing frozen water-filled icepacks into a thermal container, they should be placed on a table or surface until the sides/walls of the ice packs show drops of water have formed on all their surfaces (no frost is observed in the surface of the ice packs) and little water inside the ice packs has noted. Depending on the ambient temperature in the room the conditioning of the ice packs make take less time or more time.
Health Care Workers should plan ahead of time the preparation of ice packs for use in the transportation of vaccines, and they should strictly follow step by step the recommendations for conditioning the ice packs to avoid freeze sensitive vaccines.
Q 5 - How many ice packs should be used in a thermal container when packing vaccines?
Each manufacturer of thermal containers, which are listed for purchase from the WHO PQS catalog, have a specific number and size of ice packs for each thermal container that should be used according to their instructions, for assuring that the maximum cold life is achieved for each type of thermal container.