The Regional Program on Bioethics strengthens the development of bioethics. We build capacity in bioethics in the region and support PAHO and Member States in the integration of bioethics in all health-related activities. For background information about the Program, visit Bioethics (Concept paper, 2012). The pillars of the Program's work are research ethics and public health ethics. To learn more about the regional progress and pending challenges, visit Bioethics: Towards the integration of ethics in health: Final report (2018). The Regional Bioethics Program also houses the PAHO ethics review committee (PAHOERC). PAHOERC reviews all research with human participants in which PAHO is involved.
1. Research ethics:
We strengthen research ethics systems to ensure that research is always ethical.
2. Public health ethics:
We integrate ethics into public health work and decision-making processes that impact on the population.
What is bioethics?
Bioethics is the discipline that studies the ethical issues that arise in different areas of health, such as public health (how should health resources be distributed in a context of scarcity? what prioritization criteria should we establish and why?), health care (what are the obligations of physicians toward their patients?) and health research (how should we conduct research with people? how do we ensure the protection of those who contribute to the advancement of science?).
Bioethics is an analytical activity based on ethical principles and criteria that seeks to guide practice in the field of health. Bioethics is not a code of precepts and, as a normative discipline, it examines what "ought to be" and not what "is" because empirical evidence that something happens does not determine that it is ethically correct.
Is not the law sufficient to determine what is ethically right? What is the difference between law and ethics?
The mere fact that the law requires something does not imply that it is ethical. The law is fundamental in determining the minimum standards to be respected for coexistence among the different members of a society. What is required by law is, however, only one dimension of ethical behavior; ethics often dictates actions that go beyond what is required by law. In fact, it is neither possible nor desirable for the law to cover the entire spectrum of the moral life of individuals or societies. It is also possible for a law to dictate an action that is not considered ethical.
Ethics as a discipline allows for continuous analysis and reflection on the law and on what the law should require. Moreover, ethics should be an underpinning of the law.
What is the difference between the tasks of the Regional Program on Bioethics and PAHO's Ethics Office?
The Regional Program on Bioethics works in bioethics as the discipline that elucidates the ethical issues that arise in the field of health.
The Ethics Office seeks to ensure that PAHO employees conduct themselves ethically in the exercise of their work activities: with professional integrity and following the Organization's rules of conduct.
What is the PAHO Ethics Review Committee (PAHOERC)?
PAHOERC is the ethics committee that reviews research with human participants that is conducted with PAHO’s technical or financial involvement, in order to ensure that it is ethical. For more information, visit: https://www.paho.org/es/comite-revision-etica-ops-pahoerc
If I need assistance in research ethics or public health ethics, can I contact the Regional Program on Bioethics?
Yes, you can write to us at bioethics@paho.org.
Where can I learn more about bioethics and keep my knowledge updated?
Subscribe to our regional networks on research ethics and public health ethics to receive news, resources, materials, and invitations to Regional Bioethics Program events.
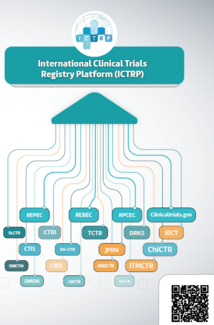
NEW INFOGRAPHIC: Clinical trial registration in ICTRP
WHO's International Clinical Trials Registry Platform (ICTRP) is a public platform providing essential information on clinical trials conducted worldwide.
BMJ Global Health Article Advancing collaborative research for health: why does collaboration matter?
NEW COURSE IN SPANISH: "Ética de la salud pública." PAHO's Regional Program on Bioethics developed this course as part of the technical cooperation with Puerto Rico. To access the course, go to the public health ethics web page in Spanish.
PUBLICATION: Tool for the accreditation of research ethics committees
Available in Spanish | Portuguese | French
Resources
- Publications and technical documents
- Ethics technical documents on COVID-19
- Webpage: Catalyzing ethical research in emergencies (NEW)
- Videos on research ethics
- Webinars & virtual events
- Regional network on ethical research
- Regional network on public health ethics
Mandates
- CD56/1NF/21 - Bioethics: Towards the integration of ethics in health: Final report (2018)
- CSP28/14, Rev. 1 (Eng.) Bioethics: Towards the integration of ethics in health. Concept paper (Sept. 2012)
- CSP28/R18 - Bioethics: Towards the integration of ethics in health. Resolution (Sept. 2012)
External Resources
Research methodology resources:
Strengthening national research ethics systems in the Americas to improve its ethics preparedness and response to emergencies (Kaleidoscope of Global Bioethics, Conselho Nacional de Ética para as Ciências da Vida, 2023)
Research integrity and responsible conduct of research (Nov. 14, 2024)
What do Research Ethics Committees review?